Table of Contents
Introduction
Hypertension after renal transplantation is a common phenomenon with an estimated prevalence of 70–90% in adults and 58–89% in children (1–3). Long-standing hypertension has been associated with allograft dysfunction, premature atherosclerosis, and cardiomyopathy (4, 5).
Cardiovascular disease (CVD) is much more common among children with chronic kidney disease (CKD) and end-stage renal disease (ESRD) compared to the general population. CVD accounts for a large proportion of morbidity and mortality in this population (6). Renal transplantation improves CVD risk. Compared to patients awaiting transplantation, transplant recipients experience a substantial reduction in the CVD-associated death rate, especially from adolescence onward while they maintain good graft function (7–11). However, hypertension remains a significant and modifiable risk factor for CVD in pediatric transplant recipients. Studies have shown that transplant patients have better systolic and diastolic function than those receiving hemodialysis or peritoneal dialysis (PD), despite having increased prevalence of hypertension and left ventricular hypertrophy (LVH) (12). In addition to offering transplantation to ameliorate the cardiovascular effects of ESRD, aggressively treating blood pressure in transplant recipients would further reduce the prevalence of CVD.
The etiology of posttransplant hypertension is multifactorial encompassing donor-associated factors, side effects of immunosuppressive medications, underlying recipient disease, and possibly genetic factors, as seen in Figure 1. The treatment strategies vary by timing after transplant as well as the pathophysiological contributors to hypertension. This review will cover etiology of posttransplant hypertension, effects of hypertension on the patient, graft survival, assessment methods, and treatment options.
Figure 1. Etiology of posttransplant hypertension.
Etiology of Hypertension
Donor Factors
It has been well established that several independent donor risk factors predispose the transplant recipient to hypertension including deceased donor, older age, and donor hypertension (13, 14). It is theorized that the increased risk comes from longer cold ischemia time, damaged graft vessels from hypertension, and age-related glomerular dropout in older donors. Due to these reasons, most transplant centers do not accept donors beyond a certain age or those with hypertension for pediatric recipients except in extenuating circumstances. Extended criteria donor kidneys have been associated with increased risk of death from CVD in the adult population and usually are not accepted for pediatric recipients (15).
The kidney donor profile index (KDPI) scoring system, used to evaluate donor grafts, was recently introduced in the United States (16). Donors with lower KDPI have potential for better graft survival, and pediatric recipients get preference for donors with lower KDPI scores, and most pediatric transplant centers will not accept kidneys with high KDPI scores for children. There are no data yet on long-term CVD outcomes for transplant recipients based on donor KDPI scores.
Recently, donor genetic variants have been found to contribute to poor graft function and posttransplant hypertension. Polymorphisms including apolipoprotein L1 (APOL1), ATP-Binding Cassette Subfamily B Member 1 (ABCB1), Caveolin 1 (CAV1), and ATP-Binding Cassette Subfamily C Member 2 (ABCC2) have been shown to affect graft survival, hypertension, and calcineurin-induced nephrotoxicity (17–21). Currently, there is no standard for screening for these polymorphisms.
Recipient Factors
Recipient factors such as recurrence of original disease in the graft and presence of native kidneys are known contributors to posttransplant hypertension. Occasionally, native nephrectomies are performed prior to or at the time of transplant to help with management of hypertension (22). However, this is becoming an uncommon practice because recent data suggest that the benefit is limited (23).
Pediatric patients with pretransplant obesity have significantly higher systolic blood pressure (SBP) and worse glomerular filtration rate (GFR) than children with normal body mass index prior to transplant (24). The incidence of pretransplant obesity is increasing mirroring the rise of obesity in the general pediatric population (25). As in the general population, the development of overweight or obesity after transplantation in children is associated with hypertension and poor glycemic control (26, 27). While these CVD risk factors can more pronounced due to the known side effects of immunosuppressant medications, the effect of obesity on hypertension in the pediatric transplant recipient has been shown to be independent of posttransplant use of steroids (28).
Effects of Medications
Immunosuppressive medications play a significant role in the development of posttransplant hypertension. It has been well established that corticosteroids induce hypertension by increasing renal salt and water reabsorption and increase in renal vascular resistance. Studies have shown that patients on steroid-minimization protocols and patients undergoing late-withdrawal of steroids showed reduction in hypertension and obesity and improved lipid and carbohydrate metabolism (29).
The incidence of posttransplant hypertension significantly increased after calcineurin inhibitors (CNI) such as cyclosporine and tacrolimus became commercially available for use. A recent Greek cohort of pediatric transplant patients showed that there was an increased risk of hypertension for patients on a CNI-based immunosuppression protocol (14).
The mechanism of CNI-induced hypertension is primarily mediated by glomerular afferent arteriolar constriction leading to increased salt and water retention, in part through upregulation of the thiazide-sensitive Na+-Cl− cotransporter (30, 31). Additional mechanisms include activation of renin angiotensin aldosterone system and secretion of reactive oxygen species (32, 33). An imbalance between vasoconstrictive molecules (endothelin, thromboxane, and prostaglandins) and vasodilatory nitric oxide also contributes to the development of hypertension (34, 35). Finally, prolonged CNI exposure causes increased production of transforming growth factor-β production, which leads to fibrosis and long-term graft damage (36–38). Tacrolimus has become the preferred CNI in clinical practice as it causes less hypertension and hyperlipidemia, as well as improved graft survival when compared to cyclosporine (39, 40).
Previously, it has been shown that mammalian target of rapamycin (mTOR) inhibitors act synergistically with CNI to worsen hypertention (41). Brunkhorst et al. (2015) recently compared the efficacy and safety of an everolimus and low-dose CSA regimen to standard dose cyclosporine and mycophenolate mofetil therapy in 105 pediatric transplant patients. They found that the everolimus group had comparable graft function and survival but more dyslipidemia and arterial hypertension than the control group (42). This suggests that mTOR inhibitors have an effect on posttransplant hypertension independent of or in synergy with the CNI effect, although the precise mechanism is not yet known.
Transplant Renal Artery Stenosis (TRAS)
Transplant renal artery stenosis is the most common vascular complication after renal transplant, usually presenting 2 months–2 years after transplant (43). Clinical signs are usually worsening or refractory hypertension with or without graft dysfunction (44). It has a reported prevalence of 1–23% and accounts for 1–5% of posttransplant hypertension (45).
Risk factors for TRAS include surgical technique, deceased donor, cytomegalovirus infection, and prolonged ischemia time (46). A recent study investigated the role of genetic polymorphisms in the myosin heavy chain 9 (MYH9) gene on TRAS. The product of this gene is heavily expressed in glomeruli, tubular, and renal capillaries as well as arteriolar endothelial cells. Donor organs found to carry the rs3752462 CC variant had a 10.9-fold increase in TRAS compared to the recipients carrying rs5756168 TT variant that had a 3.45-fold decrease in risk (47).
Graft Dysfunction
Recurrent and De Novo Glomerular Diseases
Many underlying glomerular diseases in the recipient can recur in the transplant allograft including focal segmental glomerulosclerosis (FSGS), atypical hemolytic uremic syndrome (aHUS), antiglomerular basement membrane disease, systemic lupus erythematosus nephritis, membranous glomerulonephritis (MGN), and membranoproliferative glomerulonephritis. Of these, the most common are aHUS and FSGS, the latter with a 30–50% recurrence risk in the first transplant and 80–100% in subsequent transplants (48). Some transplant patients develop de novo glomerular disorders after transplant that may include FSGS and MGN. These glomerular disorders have the potential to cause hypertension, and poor blood pressure control will accelerate renal function decline (49–51).
Acute Rejection (AR)
Acute rejection can be T-cell and antibody mediated and is often associated with hypertension. Any transplant patient with sudden onset or worsening of hypertension should be assessed for AR. The hypertension in this case usually responds well to treatment of AR.
Chronic Rejection, Also Known As Chronic Transplant Glomerulopathy (TG)
Transplant glomerulopathy causes hypertension through progressive scarring and fibrosis. Recent data showed that non-HLA autoantibodies targeting the angiotensin II type-1 receptor have been linked to hypertension, and treatment with an angiotensin receptor blocker may be beneficial (52–54).
Common Risk Factors Prevalent in the General Population
While patients with ESRD have numerous additional risk factors compared to normal children for developing hypertension, common risk factors for hypertension present in the general population also prevail in kidney transplant recipients. These risk factors include tobacco smoking, illicit drug use, medications for attention deficit hyperactivity disorder, high-salt diet, physical inactivity, obesity, sleep disturbances including obstructive sleep apnea, and genetic determinants of hypertension (55–59). Figure 2 outlines the common etiologies observed for post-transplant hypertension in relation to time after transplant.
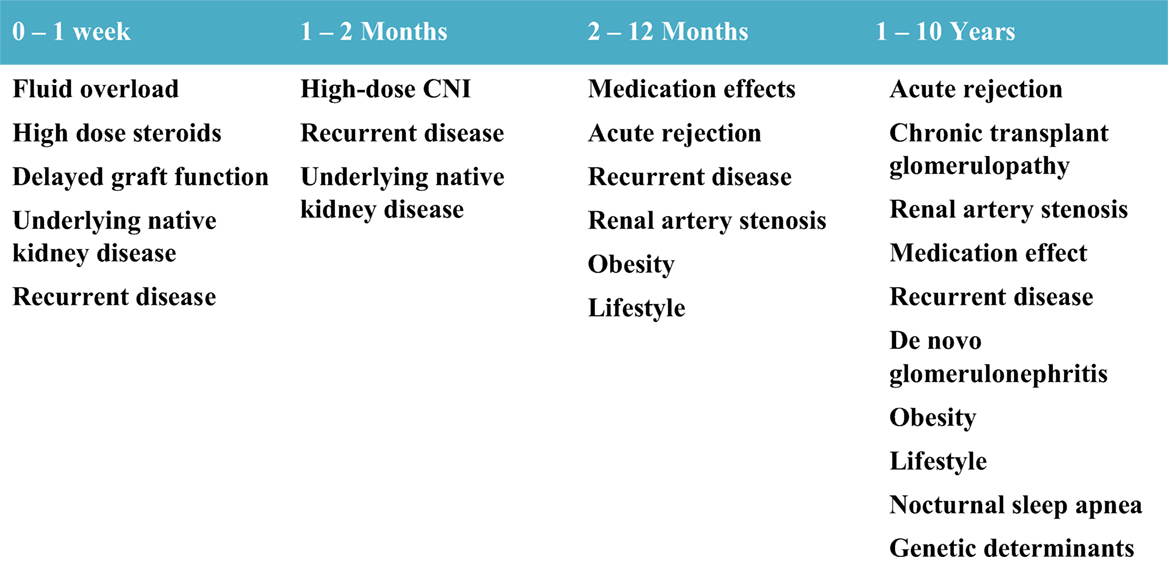
Figure 2. Etiology of hypertension by time after transplant.
Effect of Hypertension on CVD and Allograft Function
Definitive cardiovascular outcomes such as stroke, myocardial infarction, and death are uncommon in the pediatric age group. Pediatric studies have relied on the use of intermediate endpoints such as LVH as measured by left ventricular mass index (LVMI), carotid intima-media thickness (cIMT), and pulse wave velocity (PWV) for CVD risk stratification. It has been well described that pediatric patients with CKD and ESRD have significantly increased cardiovascular morbidity and mortality compared to their healthy peers (60, 61). CVD accounts for approximately 30% of mortality among pediatric kidney transplant recipients (9).
Long-standing hypertension has been shown to result in LVH and increased LVMI and cIMT, all known risk factors for CVD (2). LVH is prevalent in approximately 50% of patients with CKD and ESRD and in kidney transplant recipients. A single-center study comparing pretransplant echocardiography (ECHO) with posttransplant ECHO showed that interval decrease in indexed SBP was the only predictor of LVMI improvement on multivariate analysis (62). A cross-sectional study in 2004 found that children with a kidney transplant were more likely to have elevated LVMI and diastolic dysfunction than healthy controls (63). However, another study found no difference in systolic or diastolic function between normotensive and hypertensive pediatric transplant recipients using the same tissue Doppler technique (2).
Evidence suggests that hypertension itself, regardless of BP level, is associated with LVH. Specifically, Hamdani et al. showed that treated hypertensive individuals with a normal blood pressure had a greater prevalence of LVH than normotensive individuals not treated with antihypertensive medications. In this same study, a large pediatric cohort of 221 patients evaluated with ambulatory blood pressure monitoring (ABPM), it was noted that patients with normal blood pressure who did not require antihypertensive medications had less allograft dysfunction than patients taking antihypertensive medications and even in those whose BP was in normal range (64). This emphasizes the point that children with pharmacologically controlled hypertension are still at an increased CVD risk compared to normotensive individuals and require close screening for intermediate risk factors and endpoints.
Masked hypertension as determined by ABPM in both patients receiving and not receiving antihypertensive therapy has an estimated prevalence of 25–35% (65). While smaller studies have not been able to find an association between masked hypertension and graft function, a recent study by Hamdani et al. showed that pediatric transplant recipients with masked hypertension had significantly worse graft function than their normotensive peers (66).
A study aimed at assessing CVD risk factors in pediatric transplant patients found that there was a negative correlation between GFR and SBP ± diastolic blood pressure (DBP) on univariate analysis. There was a noted trend of worsening hypertension with increasingly poor graft function on multivariate analysis, but the study was underpowered to detect this effect (67). A Finnish cohort found that only decreased diastolic dipping could predict lower GFR (68). More recently in a Greek cohort, 20-year graft survival was superior for patients without hypertension at 10 years follow-up after kidney transplant compared to those with hypertension (100 vs. 44.4%, P < 0.05) (14). As well established in native kidneys with CKD, the presence of poorly controlled hypertension also negatively affects renal graft function over time.
A Dutch cohort study looking at long-term CVD outcomes in kidney transplant recipients (with an average follow-up of 15. 5 years) who developed ESRD as children (0–14 years of age) found that ~50% of males and 40% of females had LVH, 13% had diastolic dysfunction, and aortic valve calcification was seen in 25% males and 12% female patients (69). Duration of PD was independently associated with development of aortic valve calcifications and diastolic dysfunction increased over time and correlated with low GFR. This study also demonstrated an era effect as patients who developed ESRD in the 1970s and 1980s had increased prevalence of CVD prior to more aggressive blood pressure control, ubiquitous use of erythropoietin-stimulating agents, frequent avoidance of aluminum, and limited use of calcium-based phosphorus binders (69). A similar recent Dutch cohort still continued to show era effects in CVD outcomes with improved management of hypertension and hyperlipidemia (70).
In conclusion, children with CKD/ESRD come to transplant with significant burden of hypertension and CVD. With transplantation, their CVD risk improves overall but may be negatively impacted by poor control of blood pressure, by decline in GFR overtime, and by the presence of hyperlipidemia, obesity, and hyperglycemia.
Blood Pressure Measurement and Assessment of CVD Risk
Screening for hypertension can be achieved by several methods that include casual clinic blood pressure measurement, home and school blood pressure measurement, and 24-h ABPM. ABPM has been shown to be the most reliable method for measuring blood pressure. It also has the added benefit of determining BP load and predicting LVH (71–73). Pediatric patients with ESRD who lack nocturnal dipping as characterized by a decrease of >10% in nighttime blood pressure compared to daytime blood pressure are deemed to be at an increased risk of LVH. However, this was not borne out in another recent study that showed that hypertensive children without ESRD who have nocturnal non-dipping have similar prevalence of LVH compared to hypertensive children without non-dipping (74, 75).
Before widespread use of ABPM, we must recognize its current limitations. Primarily, controversy remains about how to best interpret ABPM results due to lack of diverse normative data. Currently available normative data are based on ABPM measurements of 949 healthy children published by the German Working Group of Pediatric Hypertension (76). All children included in this study were of European descent, relatively few short children (<140 cm) were included, and there was a lack of variability in DBPs within the group raising question about the algorithm used to calculate the normal values.
Carotid Intimal Media Thickness
High-resolution ultrasonography provides a non-invasive method for measuring cIMT. Increased cIMT, a marker for the development and progression of vascular calcification, has been documented in children as young as 8 years and correlates with duration of CKD, time on dialysis, hyperhomocysteinemia, and increased calcium–phosphorus product (77, 78). A recent meta-analysis showed that pediatric solid organ transplant recipients have increased cIMT compared to healthy controls (79). Increased cIMT has been shown to be a strong risk factor for myocardial infarction and stroke in adults (80). Childhood hypertension has been shown to predict increased adult cIMT by the Childhood Cardiovascular Cohort (i3C) (81).
Hypertension has been shown to have a linear correlation with cIMT in adults (82). Balzano et al. showed that although renal transplant recipients had increased cIMT and LVMI compared to healthy controls, there was no progression or worsening in those who underwent annual ABPM monitoring and had well-controlled hypertension (83). This study is reassuring of the fact that while renal transplant recipients have greater cIMT and LVH than healthy controls, regular blood pressure monitoring and aggressive blood pressure control can halt the progression of these intermediate CVD outcomes.
Pulse Wave Velocity
Arterial PWV is a sensitive marker of arterial stiffness, which makes it a good surrogate endpoint for CVD (84). Increased arterial stiffness is an independent predictor of survival in the general population and in CKD patients (85). Normative pediatric reference values are available based on a study of 1,000 healthy children, which enabled the calculation of age- and height-specific SD scores (86). This study was performed on patients in Hungary, Italy, and Algeria with ages ranging between 6 and 20 years. Multiple regression analysis showed that age, height, and blood pressure were major predictors of PWV.
Chronic kidney disease patients have been shown to have increased PWV. The recent 4C Study looked at numerous cardiovascular endpoints including PWV and found that 20.1% of CKD patients in their cohort had elevated PWV. In their analysis, the rise in PWV was independent of eGFR and moderately correlated with cIMT (87). Sinha et al. (2015) showed that in children with advanced CKD, only poorly controlled hypertension but not GFR was associated with a change in arterial stiffness when compared to healthy matched controls (88). In addition, the Young Finn study showed that childhood hypertension was related to higher adult PWV (89). In pediatric kidney transplant recipients, PWV has been shown to be increased compared to control patients matched for age and weight/height (26, 90). Since pediatric kidney transplant recipients have ongoing CKD, strict BP control may help to lower PWV and future cardiovascular risk in this population.
Myocardial Strain Analysis
The majority of coronary artery disease in CKD patients is asymptomatic and may initially present with arrhythmia and sudden death. Assessing the degree of myocardial strain using ECHO or magnetic resonance (MR) imaging can detect more subtle changes of impaired cardiovascular function, thus offering an opportunity for early intervention.
Echocardiographic strain imaging helps to objectively quantify myocardial function (91). An extension of this is global longitudinal strain (GLS) analysis, which can be used to detect subtle changes in left ventricular function. GLS has been shown to be independently associated with all-cause and CV mortality in adult patients with CKD and after renal transplant. It is a more reliable marker than ejection fraction for mortality (92, 93). Pirat et al. demonstrated that ESRD patients with a normal ejection fraction had signs of subclinical myocardial disease as determined by impaired longitudinal, circumferential, and radial strain as well as strain rate (94). In that same study, patients who underwent renal transplantation had improvement in all of these parameters compared to the patients receiving chronic dialysis. The clinical characteristics that negatively affect GLS and basal longitudinal systolic strain were shown to include increased interventricular septal thickness, diabetes, low ejection fraction, increased DBP, and regular dialysis (95). This again highlights the role of hypertension as a modifiable risk factor.
Cardiac MR is a relatively newer imaging modality that can provide additional information about CV risks in the CKD population. Blood oxygen level-dependent cardiac MR can be used to assess myocardial tissue oxygenation. A recent study employing this technique found that patients with CKD and renal transplant without known coronary artery disease had impaired myocardial response to stress, independent of the presence of diabetes mellitus, LVH, and myocardial scar (96). An additional use for cardiac MR imaging is to estimate LVM, which has been shown to closely correlate with autopsy findings of heart size. A recent study compared ECHO and cardiac MR in their ability to measure LVM in hypertensive pediatric patients and found that ECHO generally overestimated the presence of LVM and cardiac MR was a much more reliable method (97).
As these technologies become more readily available and cost-effective in clinical practice, they will be able to offer more reliable tools to assess cardiovascular dysfunction in pediatric transplant recipients.
Management of Hypertension
Acute Postoperative Management
There are no published guidelines or recommendations for the immediate postoperative management of kidney transplant recipients. We will describe our center’s practice based on our clinical experience in this section. In the immediate postoperative period (first week), the most likely etiology of hypertension is fluid overload, side effect of high-dose steroid therapy for induction, and native kidney disease. In the first few postoperative days, permissive hypertension is tolerated to ensure adequate graft perfusion especially in small children. Size discrepancy of the donor and recipient arteries and relatively lower blood pressure in the child can compromise early graft perfusion and predisposition to thrombus formation. This necessitates higher patient blood pressures and may require vasopressor support to achieve this.
After a few days postoperatively, excess fluid begins to mobilize and move into the intravascular space, contributing to hypertension. Allowing gradual diuresis back to the patient’s dry weight will often result in decrease of the systemic blood pressure. However, patients frequently need to be restarted or initiated on antihypertensive medications during the postoperative period.
Long-term Management
A holistic approach to the long-term management of hypertension should be pursued in the pediatric transplant recipient. As the goal of hypertensive management is ultimately to prolong patient and graft survival and decrease CVD, this approach should include close monitoring and management of graft function as well as adherence to medications, diet, and exercise. Many review papers have been published on the pharmacologic management of hypertension (1, 98, 99). The current KDIGO position is that no class of antihypertensive medications is contraindicated in transplant recipients (100). In clinical practice, calcium channel blockers (CCBs) and ACE-I are the most common first-line medications (101–103). While permissive hypertension is tolerated immediately after transplant surgery, the ultimate goal of therapy should be to target normal blood pressures (<90th percentile) and try to achieve control near the 50th percentile for age.
As with hypertension in patients without renal transplant, pharmacologic therapy should be aimed at addressing the underlying etiology. In patients on CNI-based immunosuppression, afferent arteriole vasoconstriction contributes to hypertension, and therefore, a use of CCBs is usually the first-line therapy due to its vasodilatory properties. CCBs have a very good safety profile and have been shown to be efficacious in this patient population for both blood pressure control and improvement of GFR compared to placebo treated patients (101). In non-transplant patients, the benefit of ACE-I in the pediatric CKD population with proteinuria has been well described (51). Less concrete evidence exists for the use of ACE-I in kidney transplant recipients. Knoll et al. performed a multicenter double-blind placebo-controlled study comparing ramipril to placebo and found no benefit in allograft survival in the treatment group. His study was underpowered to detect a difference and may account for their null results (104). A recent meta-analysis to look at the effects of ACE-I on graft survival also found no benefit (105). A small case series in a pediatric population found that ACE-I appeared to stabilize creatinine in patients with chronic allograft dysfunction, but statistical analysis could not support the renoprotective effect of ACE-I on long-term graft survival (106). Meta-analysis by Cross et al. showed that therapy with ACE-I can reduce proteinuria, however there are not enough data to support that this improves graft survival (101). Diuretics are not usually employed in the pediatric transplant population due to the need to maintain adequate hydration; however, a thiazide diuretic may be effective in helping control CNI-mediated hypertension due to its action through the thiazide-sensitive Na-Cl channel (30, 107).
Dietary Counseling
While pharmacological interventions have an important role in the management of hypertension, dietary and exercise counseling need to be at the forefront of long-term management of transplant recipients.
Diets high in sodium and low in potassium have been shown to aggravate hypertension. In a Belgian study of adult patients, dietary history and urinary sodium and potassium excretion were evaluated. While sodium intake did not differ between the two groups (~10 g/day), patients with controlled blood pressure consumed higher amounts of potassium by regularly eating more fruits and vegetables, which lowered their observed Na+/K+ ratio (108). Asai et al. demonstrated that repeated dietary counseling resulted in a statistically significant reduction in mean 24-h urinary sodium excretion and SBP (109). Similarly, de Vries et al. also showed that dietary sodium restriction in adult kidney transplant recipients resulted in statistically significant reductions in SBP and DBP (110). Both of these studies had small sample size, and therefore, it is impressive that the noted changes in blood pressure were able to reach statistical significance.
In accordance with these findings, published nutritional guidelines for renal transplant recipients include low-sodium diet and weight loss in obese individuals to optimize blood pressure management (111). Counseling of patients should be done routinely, as infrequent counseling has been shown to be not effective (112).
Systems-Based Approach
Changes and improvement in management of hypertension in the pediatric kidney transplant population can be approached using quality improvement methodology. Because this is a complex multifaceted problem that affects patients’ long-term outcomes and requires multidisciplinary cooperation, this can be addressed by employing the Chronic Care Model as suggested by Hooper and Mitsnefes (113). Recommendations include implementation of clinic visit checklists to ensure that blood pressure, diet, and lifestyle risk factors are addressed regularly at every clinic visit, augmenting the electronic health record (EHR) to calculate blood pressure percentiles, pop-up reminders for annual ABPM screening, and employing novel technology to encourage activity and participation among transplant recipients.
Conclusion
Hypertension in the pediatric kidney transplant recipients is highly prevalent and often underrecognized and undertreated. This contributes to worse allograft function and long-term risk of CVD in early adulthood and partly accounts for the significantly increased mortality in this patient population. Hypertension is a modifiable risk factor that should be aggressively monitored and treated, as summarized in Figure 3. Annual 24-h ABPM and echocardiography are useful tools to asses CVD risk and monitor blood pressure control. Newer technologies such as measurement of cIMT, PWV, and myocardial strain can be considered to enhance CVD risk stratification. Treatment should include a combination of pharmacological and lifestyle interventions to optimize care utilizing a multidisciplinary team. Currently, quality improvement methodology and EHR technology provide an opportunity to customize workflows and build in templates/reminders to optimize patient care and improve patient outcomes.

Figure 3. Key points.
Future Research
There are many gaps in our knowledge in this field that would benefit from further research including but not limited to CVD outcomes based on the donor KDPI; genetic polymorphism of donors affecting posttransplant hypertension in transplant recipients; ABPM normative data of diverse ethnic populations; blood pressure percentile treatment goals for transplant recipients; effect of ACE-I on long-term graft survival, and finally, addressing barriers to adherence at both provider and patient level with adequate treatment plan.
Author Contributions
OC wrote the manuscript under guidance and supervision by AM. Both parties were directly involved and were the only contributors to the production of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. Basiratnia M, Esteghamati M, Ajami GH, Amoozgar H, Cheriki C, Soltani M, et al. Blood pressure profile in renal transplant recipients and its relation to diastolic function: tissue Doppler echocardiographic study. Pediatr Nephrol (2011) 26(3):449–57. doi:10.1007/s00467-010-1724-6
3. Dobrowolski LC, van Huis M, van der Lee JH, Peters Sengers H, Lilien MR, Cransberg K, et al. Epidemiology and management of hypertension in paediatric and young adult kidney transplant recipients in The Netherlands. Nephrol Dial Transplant (2016) 31(11):1947–56. doi:10.1093/ndt/gfw225
5. Mitsnefes MM, Khoury PR, McEnery PT. Early posttransplantation hypertension and poor long-term renal allograft survival in pediatric patients. J Pediatr (2003) 143(1):98–103. doi:10.1016/S0022-3476(03)00209-9
6. Wilson AC, Mitsnefes MM. Cardiovascular disease in CKD in children: update on risk factors, risk assessment, and management. Am J Kidney Dis (2009) 54(2):345–60. doi:10.1053/j.ajkd.2009.04.027
7. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States renal data system. J Am Soc Nephrol (2007) 18(10):2644–8. doi:10.1681/ASN.2007020220
8. Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant (2004) 4(10):1662–8. doi:10.1111/j.1600-6143.2004.00573.x
9. Foster BJ, Dahhou M, Zhang X, Platt RW, Hanley JA. Change in mortality risk over time in young kidney transplant recipients. Am J Transplant (2011) 11(11):2432–42. doi:10.1111/j.1600-6143.2011.03691.x
11. Koshy SM, Guttmann A, Hebert D, Parkes RK, Logan AG. Incidence and risk factors for cardiovascular events and death in pediatric renal transplant patients: a single center long-term outcome study. Pediatr Transplant (2009) 13(8):1027–33. doi:10.1111/j.1399-3046.2008.01111.x
12. Virga G, Stomaci B, Munaro A, Mastrosimone S, Cara M, Artuso E, et al. Systolic and diastolic function in renal replacement therapy: a cross-sectional study. J Nephrol (2006) 19(2):155–60.
13. Mangray M, Vella JP. Hypertension after kidney transplant. Am J Kidney Dis (2011) 57(2):331–41. doi:10.1053/j.ajkd.2010.10.048
14. Stabouli S, Printza N, Dotis J, Gkogka C, Kollios K, Kotsis V, et al. Long-term changes in blood pressure after pediatric kidney transplantation. Am J Hypertens (2016) 29(7):860–5. doi:10.1093/ajh/hpv192
15. Blanca L, Jimenez T, Cabello M, Sola E, Gutierrez C, Burgos D, et al. Cardiovascular risk in recipients with kidney transplants from expanded criteria donors. Transplant Proc (2012) 44(9):2579–81. doi:10.1016/j.transproceed.2012.09.086
17. Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant (2011) 11(5):1025–30. doi:10.1111/j.1600-6143.2011.03513.x
18. Freedman BI, Pastan SO, Israni AK, Schladt D, Julian BA, Gautreaux MD, et al. APOL1 genotype and kidney transplantation outcomes from deceased African American donors. Transplantation (2016) 100(1):194–202. doi:10.1097/TP.0000000000000969
19. Moore J, McKnight AJ, Dohler B, Simmonds MJ, Courtney AE, Brand OJ, et al. Donor ABCB1 variant associates with increased risk for kidney allograft failure. J Am Soc Nephrol (2012) 23(11):1891–9. doi:10.1681/ASN.2012030260
20. Grisk O, Steinbach AC, Ciecholewski S, Schluter T, Kloting I, Schmidt H, et al. Multidrug resistance-related protein 2 genotype of the donor affects kidney graft function. Pharmacogenet Genomics (2009) 19(4):276–88. doi:10.1097/FPC.0b013e328328d4e9
21. Palanisamy A, Reeves-Daniel AM, Freedman BI. The impact of APOL1, CAV1, and ABCB1 gene variants on outcomes in kidney transplantation: donor and recipient effects. Pediatr Nephrol (2014) 29(9):1485–92. doi:10.1007/s00467-013-2531-7
22. Fraser N, Lyon PC, Williams AR, Christian MT, Shenoy MU. Native nephrectomy in pediatric transplantation – less is more! J Pediatr Urol (2013) 9(1):84–9. doi:10.1016/j.jpurol.2011.12.008
23. Cavallini M, Di Zazzo G, Giordano U, Pongiglione G, Dello Strologo L, Capozza N, et al. Long-term cardiovascular effects of pre-transplant native kidney nephrectomy in children. Pediatr Nephrol (2010) 25(12):2523–9. doi:10.1007/s00467-010-1638-3
24. Mitsnefes MM, Khoury P, McEnery PT. Body mass index and allograft function in pediatric renal transplantation. Pediatr Nephrol (2002) 17(7):535–9. doi:10.1007/s00467-002-0863-9
25. Hanevold CD, Ho PL, Talley L, Mitsnefes MM. Obesity and renal transplant outcome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics (2005) 115(2):352–6. doi:10.1542/peds.2004-0289
26. Degi AA, Kis E, Kerti A, Cseprekal O, Szabo AJ, Reusz GS. Prevalence of obesity and metabolic changes after kidney transplantation: Hungarian pediatric cohort study. Transplant Proc (2014) 46(6):2160–3. doi:10.1016/j.transproceed.2014.05.060
27. John EG, Domingo LT. Hypertension and obesity after pediatric kidney transplantation: management based on pathophysiology: a mini review. Int J Prev Med (2014) 5(Suppl 1):S25–38.
28. Denburg MR, Pradhan M, Shults J, Jones A, Palmer JA, Baluarte HJ, et al. Longitudinal relations between obesity and hypertension following pediatric renal transplantation. Pediatr Nephrol (2010) 25(10):2129–39. doi:10.1007/s00467-010-1572-4
29. Hocker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Improved growth and cardiovascular risk after late steroid withdrawal: 2-year results of a prospective, randomised trial in paediatric renal transplantation. Nephrol Dial Transplant (2010) 25(2):617–24. doi:10.1093/ndt/gfp506
30. Rojas-Vega L, Jimenez-Vega AR, Bazua-Valenti S, Arroyo-Garza I, Jimenez JV, Gomez-Ocadiz R, et al. Increased phosphorylation of the renal Na+-Cl− cotransporter in male kidney transplant recipient patients with hypertension: a prospective cohort. Am J Physiol Renal Physiol (2015) 309(10):F836–42. doi:10.1152/ajprenal.00326.2015
31. English J, Evan A, Houghton DC, Bennett WM. Cyclosporine-induced acute renal dysfunction in the rat. Evidence of arteriolar vasoconstriction with preservation of tubular function. Transplantation (1987) 44(1):135–41. doi:10.1097/00007890-198707000-00027
32. Nishiyama A, Kobori H, Fukui T, Zhang GX, Yao L, Rahman M, et al. Role of angiotensin II and reactive oxygen species in cyclosporine A-dependent hypertension. Hypertension (2003) 42(4):754–60. doi:10.1161/01.HYP.0000085195.38870.44
34. Gossmann J, Radounikli A, Bernemann A, Schellinski O, Raab HP, Bickeboller R, et al. Pathophysiology of cyclosporine-induced nephrotoxicity in humans: a role for nitric oxide? Kidney Blood Press Res (2001) 24(2):111–5. doi:10.1159/000054216
35. Gonzalez-Santiago L, Lopez-Ongil S, Lamas S, Quereda C, Rodriguez-Puyol M, Rodriguez-Puyol D. Imbalance in endothelial vasoactive factors as a possible cause of cyclosporin toxicity: a role for endothelin-converting enzyme. J Lab Clin Med (2000) 136(5):395–401. doi:10.1067/mlc.2000.110370
36. Bennett J, Cassidy H, Slattery C, Ryan MP, McMorrow T. Tacrolimus modulates TGF-beta signaling to induce epithelial-mesenchymal transition in human renal proximal tubule epithelial cells. J Clin Med (2016) 5(5):50. doi:10.3390/jcm5050050
37. Rivelli RF, Goncalves RT, Leite M Jr, Santos MA, Delgado AG, Cardoso LR, et al. Early withdrawal of calcineurin inhibitor from a sirolimus-based immunosuppression stabilizes fibrosis and the transforming growth factor-beta signalling pathway in kidney transplant. Nephrology (Carlton) (2015) 20(3):168–76. doi:10.1111/nep.12368
38. Alpay N, Ozkok A, Caliskan Y, Akagun T, Cinar SA, Deniz G, et al. Influence of conversion from calcineurin inhibitors to everolimus on fibrosis, inflammation, tubular damage and vascular function in renal transplant patients. Clin Exp Nephrol (2014) 18(6):961–7. doi:10.1007/s10157-014-0939-4
39. Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation (2002) 73(5):775–82. doi:10.1097/00007890-200203150-00021
40. Margreiter R; European Tacrolimus vs Ciclosporin Microemulsion Renal Transplantation Study Group. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study. Lancet (2002) 359(9308):741–6. doi:10.1016/S0140-6736(02)07875-3
41. Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation (2003) 75(8):1213–20. doi:10.1097/01.TP.0000062837.99400.60
42. Brunkhorst LC, Fichtner A, Hocker B, Burmeister G, Ahlenstiel-Grunow T, Krupka K, et al. Efficacy and safety of an everolimus- vs. a mycophenolate mofetil-based regimen in pediatric renal transplant recipients. PLoS One (2015) 10(9):e0135439. doi:10.1371/journal.pone.0135439
43. Chen W, Kayler LK, Zand MS, Muttana R, Chernyak V, DeBoccardo GO. Transplant renal artery stenosis: clinical manifestations, diagnosis and therapy. Clin Kidney J (2015) 8(1):71–8. doi:10.1093/ckj/sfu132
44. Srivastava A, Kumar J, Sharma S, Abhishek, Ansari MS, Kapoor R. Vascular complication in live related renal transplant: an experience of 1945 cases. Indian J Urol (2013) 29(1):42–7. doi:10.4103/0970-1591.109983
46. Patel NH, Jindal RM, Wilkin T, Rose S, Johnson MS, Shah H, et al. Renal arterial stenosis in renal allografts: retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology (2001) 219(3):663–7. doi:10.1148/radiology.219.3.r01jn30663
47. Pazik J, Lewandowski Z, Oldak M, Ozieblo D, Perkowska Ptasinska A, Sadowska A, et al. Association of MYH9 rs3752462 and rs5756168 polymorphisms with transplanted kidney artery stenosis. Transplant Proc (2016) 48(5):1561–5. doi:10.1016/j.transproceed.2016.01.085
48. Ponticelli C. Recurrence of focal segmental glomerular sclerosis (FSGS) after renal transplantation. Nephrol Dial Transplant (2010) 25(1):25–31. doi:10.1093/ndt/gfp538
49. Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension (2008) 52(4):631–7. doi:10.1161/HYPERTENSIONAHA.108.110635
50. Warady BA, Abraham AG, Schwartz GJ, Wong CS, Munoz A, Betoko A, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis (2015) 65(6):878–88. doi:10.1053/j.ajkd.2015.01.008
51. Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med (2009) 361(17):1639–50. doi:10.1056/NEJMoa0902066
52. Dragun D. The role of angiotensin II type 1 receptor-activating antibodies in renal allograft vascular rejection. Pediatr Nephrol (2007) 22(7):911–4. doi:10.1007/s00467-007-0452-z
53. Wei F, Jia XJ, Yu SQ, Gu Y, Wang L, Guo XM, et al. Candesartan versus imidapril in hypertension: a randomised study to assess effects of anti-AT1 receptor autoantibodies. Heart (2011) 97(6):479–84. doi:10.1136/hrt.2009.192104
54. Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med (2005) 352(6):558–69. doi:10.1056/NEJMoa035717
55. Rao G. Diagnosis, epidemiology, and management of hypertension in children. Pediatrics (2016) 138(2):e20153616. doi:10.1542/peds.2015-3616
56. Magnussen CG, Smith KJ, Juonala M. When to prevent cardiovascular disease? As early as possible: lessons from prospective cohorts beginning in childhood. Curr Opin Cardiol (2013) 28(5):561–8. doi:10.1097/HCO.0b013e32836428f4
58. Martinez-Raga J, Knecht C, Szerman N, Martinez MI. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs (2013) 27(1):15–30. doi:10.1007/s40263-012-0019-9
59. Mick E, McManus DD, Goldberg RJ. Meta-analysis of increased heart rate and blood pressure associated with CNS stimulant treatment of ADHD in adults. Eur Neuropsychopharmacol (2013) 23(6):534–41. doi:10.1016/j.euroneuro.2012.06.011
62. Mitsnefes MM, Schwartz SM, Daniels SR, Kimball TR, Khoury P, Strife CF. Changes in left ventricular mass index in children and adolescents after renal transplantation. Pediatr Transplant (2001) 5(4):279–84. doi:10.1034/j.1399-3046.2001.005004279.x
63. Mitsnefes MM, Kimball TR, Border WL, Witt SA, Glascock BJ, Khoury PR, et al. Abnormal cardiac function in children after renal transplantation. Am J Kidney Dis (2004) 43(4):721–6. doi:10.1053/j.ajkd.2003.12.033
64. Hamdani G, Nehus EJ, Hanevold CD, Sebestyen Van Sickle J, Woroniecki R, Wenderfer SE, et al. Ambulatory blood pressure, left ventricular hypertrophy, and allograft function in children and young adults after kidney transplantation. Transplantation (2017) 101(1):150–6. doi:10.1097/TP.0000000000001087
65. Paripovic D, Kostic M, Spasojevic B, Kruscic D, Peco-Antic A. Masked hypertension and hidden uncontrolled hypertension after renal transplantation. Pediatr Nephrol (2010) 25(9):1719–24. doi:10.1007/s00467-010-1552-8
66. Hamdani G, Nehus EJ, Hooper DK, Mitsnefes MM. Masked hypertension and allograft function in pediatric and young adults kidney transplant recipients. Pediatr Transplant (2016) 20(8):1026–31. doi:10.1111/petr.12752
67. Silverstein DM, Mitchell M, LeBlanc P, Boudreaux JP. Assessment of risk factors for cardiovasuclar disease in pediatric renal transplant patients. Pediatr Transplant (2007) 11(7):721–9. doi:10.1111/j.1399-3046.2007.00730.x
68. Tainio J, Qvist E, Miettinen J, Holtta T, Pakarinen M, Jahnukainen T, et al. Blood pressure profiles 5 to 10 years after transplant in pediatric solid organ recipients. J Clin Hypertens (Greenwich) (2015) 17(2):154–61. doi:10.1111/jch.12465
69. Gruppen MP, Groothoff JW, Prins M, van der Wouw P, Offringa M, Bos WJ, et al. Cardiac disease in young adult patients with end-stage renal disease since childhood: a Dutch cohort study. Kidney Int (2003) 63(3):1058–65. doi:10.1046/j.1523-1755.2003.00814.x
70. Vogelzang JL, Heestermans LW, van Stralen KJ, Jager KJ, Groothoff JW. Simultaneous reversal of risk factors for cardiac death and intensified therapy in long-term survivors of paediatric end-stage renal disease over the last 10 years. Nephrol Dial Transplant (2013) 28(10):2545–52. doi:10.1093/ndt/gft257
71. Stabouli S, Kotsis V, Rizos Z, Toumanidis S, Karagianni C, Constantopoulos A, et al. Left ventricular mass in normotensive, prehypertensive and hypertensive children and adolescents. Pediatr Nephrol (2009) 24(8):1545–51. doi:10.1007/s00467-009-1165-2
72. Richey PA, Disessa TG, Hastings MC, Somes GW, Alpert BS, Jones DP. Ambulatory blood pressure and increased left ventricular mass in children at risk for hypertension. J Pediatr (2008) 152(3):343–8. doi:10.1016/j.jpeds.2007.07.014
73. Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension (2002) 39(4):903–8. doi:10.1161/01.HYP.0000013266.40320.3B
74. Chaudhuri A, Sutherland SM, Begin B, Salsbery K, McCabe L, Potter D, et al. Role of twenty-four-hour ambulatory blood pressure monitoring in children on dialysis. Clin J Am Soc Nephrol (2011) 6(4):870–6. doi:10.2215/CJN.07960910
75. Seeman T, Hradsky O, Gilik J. Nocturnal blood pressure non-dipping is not associated with increased left ventricular mass index in hypertensive children without end-stage renal failure. Eur J Pediatr (2016) 175(8):1091–7. doi:10.1007/s00431-016-2749-z
76. Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F; German Working Group on Pediatric Hypertension. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens (2002) 20(10):1995–2007. doi:10.1097/00004872-200210000-00019
77. Bilginer Y, Ozaltin F, Basaran C, Aki TF, Karabulut E, Duzova A, et al. Carotid intima-media thickness in children and young adults with renal transplant: internal carotid artery vs. common carotid artery. Pediatr Transplant (2007) 11(8):888–94. doi:10.1111/j.1399-3046.2007.00760.x
78. Delucchi A, Dinamarca H, Gainza H, Whitttle C, Torrealba I, Iniguez G. Carotid intima-media thickness as a cardiovascular risk marker in pediatric end-stage renal disease patients on dialysis and in renal transplantation. Transplant Proc (2008) 40(9):3244–6. doi:10.1016/j.transproceed.2008.03.126
79. Al Nasser Y, Moura MC, Mertens L, McCrindle BW, Parekh RS, Ng VL, et al. Subclinical cardiovascular changes in pediatric solid organ transplant recipients: a systematic review and meta-analysis. Pediatr Transplant (2016) 20(4):530–9. doi:10.1111/petr.12689
80. O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med (1999) 340(1):14–22. doi:10.1056/NEJM199901073400103
81. Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation (2010) 122(24):2514–20. doi:10.1161/CIRCULATIONAHA.110.966465
82. Ferreira JP, Girerd N, Bozec E, Machu JL, Boivin JM, London GM, et al. Intima-media thickness is linearly and continuously associated with systolic blood pressure in a population-based cohort (STANISLAS Cohort Study). J Am Heart Assoc (2016) 5(6):e003529. doi:10.1161/JAHA.116.003529
83. Balzano R, Lindblad YT, Vavilis G, Jogestrand T, Berg UB, Krmar RT. Use of annual ABPM, and repeated carotid scan and echocardiography to monitor cardiovascular health over nine yr in pediatric and young adult renal transplant recipients. Pediatr Transplant (2011) 15(6):635–41. doi:10.1111/j.1399-3046.2011.01547.x
86. Reusz GS, Cseprekal O, Temmar M, Kis E, Cherif AB, Thaleb A, et al. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension (2010) 56(2):217–24. doi:10.1161/HYPERTENSIONAHA.110.152686
87. Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, et al. Cardiovascular phenotypes in children with CKD: the 4C study. Clin J Am Soc Nephrol (2017) 12(1):19–28. doi:10.2215/CJN.01090216
88. Sinha MD, Keehn L, Milne L, Sofocleous P, Chowienczyk PJ. Decreased arterial elasticity in children with nondialysis chronic kidney disease is related to blood pressure and not to glomerular filtration rate. Hypertension (2015) 66(4):809–15. doi:10.1161/HYPERTENSIONAHA.115.05516
89. Koivistoinen T, Hutri-Kahonen N, Juonala M, Aatola H, Koobi T, Lehtimaki T, et al. Metabolic syndrome in childhood and increased arterial stiffness in adulthood: the Cardiovascular Risk In Young Finns Study. Ann Med (2011) 43(4):312–9. doi:10.3109/07853890.2010.549145
90. Cseprekal O, Kis E, Schaffer P, Othmane Tel H, Fekete BC, Vannay A, et al. Pulse wave velocity in children following renal transplantation. Nephrol Dial Transplant (2009) 24(1):309–15. doi:10.1093/ndt/gfn494
92. Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Leano R, Haluska BA, et al. The association between left ventricular global longitudinal strain, renal impairment and all-cause mortality. Nephrol Dial Transplant (2014) 29(6):1218–25. doi:10.1093/ndt/gfu004
93. Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Burrage M, Leano R, et al. Left ventricular global longitudinal strain (GLS) is a superior predictor of all-cause and cardiovascular mortality when compared to ejection fraction in advanced chronic kidney disease. PLoS One (2015) 10(5):e0127044. doi:10.1371/journal.pone.0127044
94. Pirat B, Bozbas H, Simsek V, Sade LE, Sayin B, Muderrisoglu H, et al. Assessment of myocardial mechanics in patients with end-stage renal disease and renal transplant recipients using speckle tracking echocardiography. Exp Clin Transplant (2015) 13(Suppl 1):235–41.
95. Rhea IB, Morris K, Sawada S, Feigenbaum H. Prevalence, etiology, and clinical implications of reduced longitudinal systolic strain in renal transplant candidates. Echocardiography (2016) 33(11):1676–82. doi:10.1111/echo.13307
96. Parnham S, Gleadle JM, Bangalore S, Grover S, Perry R, Woodman RJ, et al. Impaired myocardial oxygenation response to stress in patients with chronic kidney disease. J Am Heart Assoc (2015) 4(8):e002249. doi:10.1161/JAHA.115.002249
97. Supe-Markovina K, Nielsen JC, Musani M, Panesar LE, Woroniecki RP. Assessment of left ventricular mass and hypertrophy by cardiovascular magnetic resonance imaging in pediatric hypertension. J Clin Hypertens (Greenwich) (2016) 18(10):976–81. doi:10.1111/jch.12808
99. Glicklich D, Lamba R, Pawar R. Hypertension in the kidney transplant recipient: overview of pathogenesis, clinical assessment and treatment. Cardiol Rev (2017) 25(3):102–9. doi:10.1097/CRD.0000000000000126
100. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant (2009) 9(Suppl 3):S1–155. doi:10.1111/j.1600-6143.2009.02834.x
101. Cross NB, Webster AC, Masson P, O’Connell PJ, Craig JC. Antihypertensives for kidney transplant recipients: systematic review and meta-analysis of randomized controlled trials. Transplantation (2009) 88(1):7–18. doi:10.1097/TP.0b013e3181a9e960
102. Dawidson I, Rooth P, Lu C, Sagalowsky A, Diller K, Palmer B, et al. Verapamil improves the outcome after cadaver renal transplantation. J Am Soc Nephrol (1991) 2(5):983–90.
103. European Best Practice Guidelines for Renal Transplantation. Section IV: long-term management of the transplant recipient. IV.5.2. Cardiovascular risks. Arterial hypertension. Nephrol Dial Transplant (2002) 17(Suppl 4):25–6.
104. Knoll GA, Fergusson D, Chasse M, Hebert P, Wells G, Tibbles LA, et al. Ramipril versus placebo in kidney transplant patients with proteinuria: a multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol (2016) 4(4):318–26. doi:10.1016/S2213-8587(15)00368-X
105. Cheungpasitporn W, Thongprayoon C, Mao MA, Kittanamongkolchai W, Sathick IJ, Erickson SB. The effect of renin-angiotensin system inhibitors on kidney allograft survival: a systematic review and meta-analysis. N Am J Med Sci (2016) 8(7):291–6. doi:10.4103/1947-2714.187141
106. Arbeiter K, Pichler A, Stemberger R, Mueller T, Ruffingshofer D, Vargha R, et al. ACE inhibition in the treatment of children after renal transplantation. Pediatr Nephrol (2004) 19(2):222–6. doi:10.1007/s00467-003-1317-8
107. Hoorn EJ, Walsh SB, McCormick JA, Furstenberg A, Yang CL, Roeschel T, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med (2011) 17(10):1304–9. doi:10.1038/nm.2497
108. Saint-Remy A, Somja M, Gellner K, Weekers L, Bonvoisin C, Krzesinski JM. Urinary and dietary sodium and potassium associated with blood pressure control in treated hypertensive kidney transplant recipients: an observational study. BMC Nephrol (2012) 13:121. doi:10.1186/1471-2369-13-121
109. Asai K, Kobayashi T, Miyata H, Tanaka Y, Okada Y, Sakai K, et al. The short-term impact of dietary counseling on sodium intake and blood pressure in renal allograft recipients. Prog Transplant (2016). doi:10.1177/1526924816664084
110. de Vries LV, Dobrowolski LC, van den Bosch JJ, Riphagen IJ, Krediet CT, Bemelman FJ, et al. Effects of dietary sodium restriction in kidney transplant recipients treated with renin-angiotensin-aldosterone system blockade: a randomized clinical trial. Am J Kidney Dis (2016) 67(6):936–44. doi:10.1053/j.ajkd.2015.11.026
111. Chan M, Patwardhan A, Ryan C, Trevillian P, Chadban S, Westgarth F, et al. Evidence-based guidelines for the nutritional management of adult kidney transplant recipients. J Ren Nutr (2011) 21(1):47–51. doi:10.1053/j.jrn.2010.10.021
112. Cameron C, Krmar RT. Single-center assessment of nutritional counseling in preventing excessive weight gain in pediatric renal transplants recipients. Pediatr Transplant (2016) 20(3):388–94. doi:10.1111/petr.12668